Energy consumption is a significant cost for most manufacturing and process plants. It’s also the primary source of greenhouse gas (GHG) emissions. When inventorying and accounting for these emissions, the source is defined as Scope 1, 2, or 3.
Scope 1 emissions are related to combusted fuels. Scope 2 emissions are related to purchased energy, such as electricity. Scope 3 emissions relate to the upstream and downstream sides of a plant’s supply chain. I will address the topic of combustion efficiency and its ability to improve energy efficiency and reduce GHG emissions.
Combustion Basics
For most industrial processes, hydrocarbons in the form of natural gas, coal, petroleum, fuel oil, and the like are combusted to, among other things, heat boilers and furnaces and fuel reciprocating engines and gas turbines. Other fuels employed in industrial processes include biomass, agricultural residues, forest products, pulping liquids (black liquor), etc.
Of the roughly 25 quadrillion British Thermal Units (BTUs) of industrial energy consumed in the United States each year, natural gas, petroleum distillates, and coal represent about 80%.
Fuels store energy in a chemical form. Chemical energy is converted to thermal and mechanical energy via combustion. Combustion is an oxidation-reduction (redox) process. Natural gas, the most popular industrial fuel, comprises about 60-90% methane (CH4).
The balance is a combination of ethane (C2H6), propane (C3H8), butane (C4H10), and some trace elements and molecules. When the combustion of methane is stoichiometric (i.e., in simple integral ratios as prescribed by an equation or ratio), one molecule of CH4 combines with two molecules of O2 to form one molecule of CO2 and molecules of H2O and, of course, heat.
When the combustion of a hydrocarbon is imperfect, intermediate products such as carbon monoxide (CO), soot, aldehydes, etc., are produced as byproducts.
Atmospheric air, not pure O2, provides the oxygen for combustion in most industrial processes. Atmospheric air typically supplies the oxygen required for that combustion.
Nitrogen, an inert gas, represents about 78% of the volume of atmospheric air and most of its mass. Oxygen, required for the combustion redox reaction, makes up only about 21% of atmospheric air’s volume and just over 23% of its mass.
The relative scarcity of oxygen in atmospheric air and the challenge of distributing the hydrocarbon molecules within the chemical matrix typically requires an over-stoichiometric mixture of air to fuel to achieve high levels of combustion efficiency. However, too much excessive air can compromise efficiency.
Getting the Air-to-Fuel Ratio Right: A Natural-Gas-Boiler Example
The United States Department of Energy (USDOE) has created various energy-efficiency-improvement guides and tips for industrial users. These guides are offered at no cost (through free downloads). One such guide addresses the relationship between excess air, flue-gas temperature, and the efficiency of natural gas-burning boilers.
As previously discussed, the challenges in evenly distributing fuel in air, combined with the fact that oxygen is a minority component of atmospheric air, require excess air (over-stochiometric) to achieve maximum efficiency. Too little or too much excess air results in reduced efficiency.
The ideal method for determining the optimum amount of excess air is flue-gas composition and temperature analysis. If flue gases contain excess fuel, soot, smoke, or carbon monoxide, the combustion process requires more excess air to achieve optimum efficiency.
Excess air must be reduced if the difference between combustion-air temperature and flue-gas temperature is excessive. The impact on combustion efficiency for a natural gas boiler can be quite dramatic, as illustrated in (Table 1).
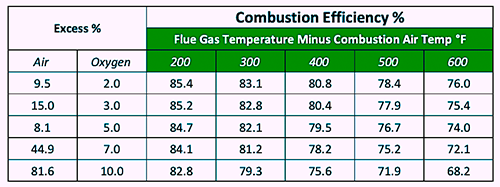
Table 1. Relationship Between Excess-Air Percentage And Flue-Gas Temperature To Boiler Efficiency (Ref: USDOE).
Suppose that a boiler uses 500,000 MMBtu of natural gas per year, operates with 44.9% excess air (7% oxygen), and its flue-gas temperature exceeds the combustion-air temperature by 400⁰ F degrees.
According to Table 1, the boiler efficiency is estimated to be 78.2%. Tuning the combustion to reduce an excess-air percentage of 9.5% (2.0% oxygen) and flue-gas temperature to 200 F degrees above the combustion-air temperature will, on average, improve the boiler’s efficiency to 85.4%.
The more efficient boiler would require 8.43% less energy to produce the same steam. That would be a reduction of 42,154 MMBtu of natural gas. Assuming a market price of US$8.50 per MMBtu, this equates to an annual fuel savings of US$358,314!
As previously discussed, combusted natural gas produces H2O and CO2 as normal oxidation byproducts. Unless captured and stored or converted, each MMBtu of combusted natural gas emits 14.43 kg of CO2 into the atmosphere. Therefore, the efficiency improvements described above would reduce Scope 1 GHG emissions by over 608 metric tons (mt) per year.
Estimates of the social cost of carbon (SCC) vary widely, but US$50 per metric ton is the most often quoted value. Assuming this value, the social benefits of improving the boiler’s efficiency in our example would result in more than US$30,000 in avoided social costs associated with GHG emissions.
The social costs of carbon include adverse health effects, increases in extreme weather patterns and natural disasters, rising sea levels, localized water shortages, food insecurity, etc.
The combustion of chemically stored fuels, such as natural gas, coal, petroleum, fuel oil, etc., represents a significant cost for most industrial plants and facilities. It’s also the primary source of most Scope 1 greenhouse gas (GHG) emissions.
Improving combustion efficiency affords asset managers a tremendous opportunity to reduce operating fuel costs, GHG emissions, and the social costs of carbon (SCC). It also reduces wear and tear on a site’s equipment.
Careful monitoring of boiler-flue-gas composition and temperature and taking appropriate measures to improve efficiency offer a solution that’s easy to justify and implement. In short, this approach makes for a big, all-around win for the organization and the environment.
Initially published in The RAM Review.










